Biopharmaceutical GMP purification workshop
Adapt to the industry:Biological experiment, drug production Suggested level: 100-300,000
(1) Requirements for biomedical GMP purification workshop
★ The goal of GMP for biopharmaceutical companies is to ensure the establishment of a scientific and rigorous sterile pharmaceutical production environment, process, operation and management system to minimize all possible and potential biological activities, dust, pyrogen pollution, and production. High-quality, hygienic and safe pharmaceutical products. We call the bio-pharmaceutical purification project - GMP clean plant engineering solutions and pollution control technology is one of the main means to ensure the successful implementation of GMP.

(two), constant standardWhat can I do for you?
★ Through in-depth research and engineering experience of biopharmaceutical customers' production environment, we clearly understand the key to environmental control of biopharmaceutical production processes;
★ Energy saving is the priority of our system plan;
★ What we are best at is to give customers environmental solutions that meet the requirements of GMP and Fed 209D, ISO14644, IEST, EN1822 and apply the latest energy-saving technologies;
★ We can provide planning and design from GMP whole plant - human flow logistics purification program, clean air conditioning system, clean decoration system
★ Complete installation and supporting services for energy-saving renovation, hydropower, ultra-pure gas pipelines, clean room monitoring and maintenance systems.

(III) Technical parameters of biomedical GMP purification workshop
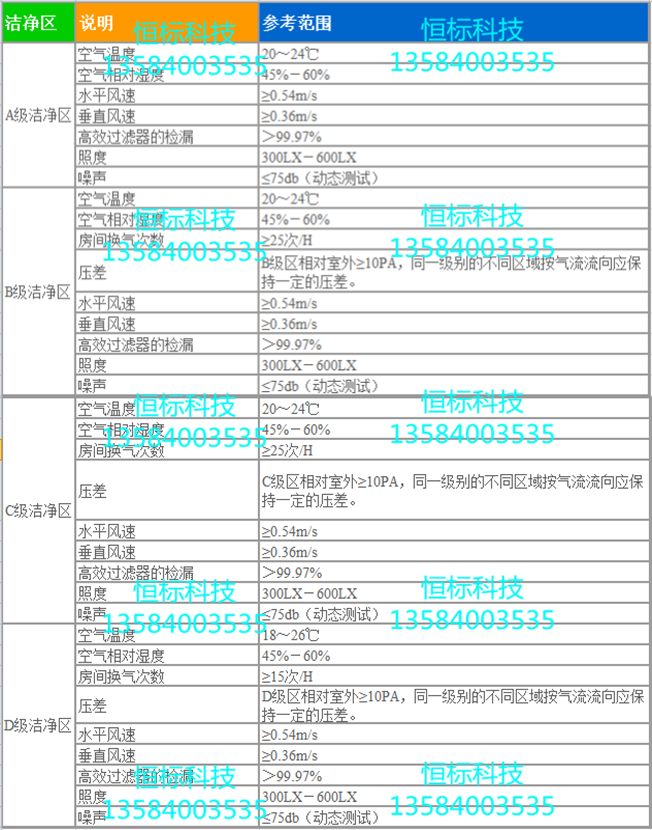
The clean room of the pharmaceutical factory is divided into four levels: A, B, C, and D. The clean room and clean area of the pharmaceutical industry are mainly controlled by particles and microorganisms. At the same time, the temperature difference between the temperature and humidity should be seen in GMP (2010). Illumination, noise, etc. are regulated. See GMP (2010) for the air cleanliness level of the clean room of the pharmaceutical industry, which is specified as four grades A, B, C and D.
Class A area: High-risk operation area, such as filling area, place rubber stopper barrel, open ampoule bottle, open vial area and aseptic assembly line or connection operation area. A laminar flow station (cover) is typically used to maintain the environmental state of the zone. The laminar flow system must deliver air evenly in its working area with a wind speed of 0.36-0.54 M/S (guide value). There should be data demonstrating the state of laminar flow and verification is required, and unidirectional flow or lower wind speed may be used in a closed isolated operating area or glove box.
Class B area: refers to the background area where the high-risk operation A-level area such as aseptic preparation and filling is located.
Class C and Class D zones: Refers to the less important clean operating areas in the production of sterile drugs.
(four),Air cleanliness standard for clean areas (rooms) in China's pharmaceutical production
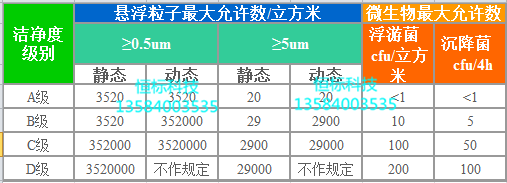
Grade A and Grade B are equivalent to 100 grades. The background environment of Grade A is higher and the requirements are stricter.
Class C is equivalent to 10,000
Class D is equivalent to 100,000
In order to confirm the level of Class A clean area, the sampling amount of each sampling point shall not be less than 1 cubic meter. The level of airborne particles in the Class A clean zone is ISO 4.8, with a limit of ≥ 5.0 μm suspended particles. Class B clean areas (static) have airborne particles of the class ISO 5 and include suspended particles of two particle sizes in the table. For Class C clean areas (static and dynamic), the levels of airborne particles are ISO 7 and ISO 8. For class D clean zone (static) airborne particles are rated ISO 8 . The test method can be referred to ISO 14644-1.
(V) Working environment requirements of the pharmaceutical unit A\B\C\D clean area